What is internal energy in daily life? It’s the hidden energy inside everything—from your morning coffee to the fridge keeping your food fresh.
In this article, you’ll see 6 simple real-life examples of internal energy (boiling water, melting ice, etc.) and learn the first law of thermodynamics ΔU = Q – W in plain English—no PhD required.
- ΔU = change in internal energy
- Q = heat added to the system (positive when absorbed)
- W = work done by the system (positive when the system expands)
Note: This uses the physics convention; in chemistry, it’s often ΔU = Q + W.
What Is Internal Energy?
Internal energy is the total microscopic energy stored within a substance due to the random motion (kinetic energy) of its molecules and the forces (potential energy) between them—independent of the system’s position or external motion.
In daily life, it’s the invisible “energy bank” that rises when you heat soup, drops when your drink cools, and shifts dramatically during melting or boiling—all governed by the first law:
ΔU = Q − W
Note: This uses the physics convention; in chemistry, it’s often ΔU = Q + W.
Science Behind Internal Energy
When a material is heated or cooled, its particles undergo two key changes. Firstly, chemical bonds may form, break, or stretch, altering the material’s chemical energy storage. Simultaneously, the material heats up or cools down as particles gain or lose speed, affecting its thermal energy storage.
Below are six real life examples of internal energy.
6 Real-Life Examples of Internal Energy (with Math)
1. Boiling Water on the Stove
What you see: Water turns from liquid to steam.
Energy flow:
- Heat
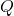 is supplied by the burner.
is supplied by the burner. - No work
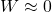 (volume change of liquid water is tiny).
(volume change of liquid water is tiny). - All heat goes into breaking intermolecular bonds → latent heat of vaporization.
Math:
![]()
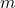 = mass of water
= mass of water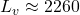 kJ/kg at 100 °C
kJ/kg at 100 °C
Example: Boil 0.5 kg of water:
![]()
Key: Temperature stays at 100 °C while U rises due to potential energy increase.

2. Melting Ice in a Glass
What you see: Ice melts into water at 0 °C.
Energy flow:
- Heat
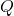 flows from the room.
flows from the room. - No work (
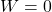 ).
). - Energy breaks hydrogen bonds → latent heat of fusion.
Math:
![]()
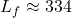 kJ/kg
kJ/kg
Example: Melt 0.1 kg of ice:
![]()
Key: Temperature stays 0 °C; U increases via potential energy.

3. Hot Coffee Cooling on the Table
What you see: Coffee cools from 80 °C to 25 °C.
Energy flow:
- Heat
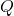 leaves (negative).
leaves (negative). - No work (
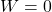 ).
).
Math:
![]()
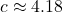 kJ/kg·°C
kJ/kg·°C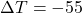 °C
°C
Example: 0.2 kg coffee:
![]()
Key: U decreases due to kinetic energy loss.
4. Rubbing Your Hands Together
What you see: Hands feel warm.
Energy flow:
- Work done on hands →
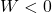
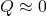
Math:
![]()
Example: 50 J work done:
![]()
Key: Work → kinetic energy → warmth.
5. Inflating a Bicycle Tire
What you see: Pressure rises.
Energy flow:
- Work done on gas →
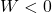
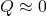
Math:
![]()
Example: 200 J work:
![]()
Key: Molecules speed up → higher kinetic energy.
6. Refrigerator Cooling Food
What you see: Food stays cold.
Energy flow (inside):
- Heat
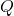 removed (
removed (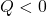 )
) 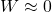
Math:
![]()
Example: 1 kg milk cools from 10 °C to 4 °C:
![]()
![]()
Key: U drops as molecules slow down.
More Exmples
| No. | Example | Process | Quick Calculation |
|---|---|---|---|
| 1 | Air Conditioner | Heat pump | 500 W × 3600 s = 1.8 MJ removed |
| 2 | Body Metabolism | Chemical → Thermal | 2000 kcal ≈ 8.4 MJ/day |
| 3 | Microwave Heating | EM → KE | 800 W × 120 s = 96 kJ |
| 4 | Car Engine | Chemical → KE | 0.01 L fuel ≈ 340 kJ |
| 5 | Sweating (Evaporation) | Cooling | 0.01 kg × 2.26 MJ/kg = 22.6 kJ lost |
| 6 | Pressure Cooker | High T, high P | ≈ 400 kJ for 1 kg water |
| 7 | Dry Ice Sublimation | Solid → Gas | 0.1 kg × 571 kJ/kg = 57 kJ |
| 8 | LPG Stove | Combustion | 0.01 kg × 46 MJ/kg = 460 kJ |
| 9 | Fridge Bulb | Joule heating | 4 W × 86 400 s = 346 kJ |
Summary Crux Points
- The internal energy is the total kinetic and potential energy of all particles in the system.
- Adding energy to raise the temperature results in particles speeding up, gaining kinetic energy.
- Melting or boiling a substance requires energy to break bonds, increasing potential energy.
- Internal energy includes both kinetic (particle speed) and potential (bond strength) components.
- The effect of energy transfer (breaking bonds, stretching bonds, or increasing particle speed) depends on temperature and material state.
